All Staff Stress and Resilience Workshop delivered by The Mind Room
In mid-November, CTA was pleased to offer a Stress and Resilience Workshop for all staff, delivered by Bailey Opie, a Performance Psychologist at The Mind Room. We were extremely [...]
CTA’s Developmental Therapeutics Update Dinner Series draws to a close for the second year
In late November CTA was pleased to host our second Developmental Therapeutics Update Dinner meeting for 2023, presented with the generous support of the Series sponsors, namely Amgen, AstraZeneca, [...]
CTA’s Annual Research Managers’ Day 2023
In late October CTA was pleased to host our Annual Research Managers’ Day, a site driven, in-person forum that continues to grow in popularity each year. In 2023 we [...]
Cancer Trials Australia Annual Report 2022
I am pleased to share with you our 2022 Annual Report, released at our recent Annual General Meeting on 24 May, 2023. This Annual Report also takes the opportunity [...]
Cancer Trials Australia Annual Report 2021
I am pleased to share with you the 2021 Cancer Trials Australia Annual Report, released at our recent Annual General Meeting on 18 May, 2022. Please click here to access [...]
CTA’s New Chairperson
Welcoming Associate Professor Jayesh Desai as the Incoming Chairperson of the Cancer Trials Australia Board of Directors As his six-year tenure draws to a conclusion, outgoing Cancer Trials [...]
Cancer Trials Australia Annual Report 2020
I am pleased to share with you the 2020 Cancer Trials Australia Annual Report, released at our recent Annual General Meeting.Please click here to access the reportAs the report describes, despite [...]
10,000th Australian patient accrued by ground-breaking cancer trials collaborative
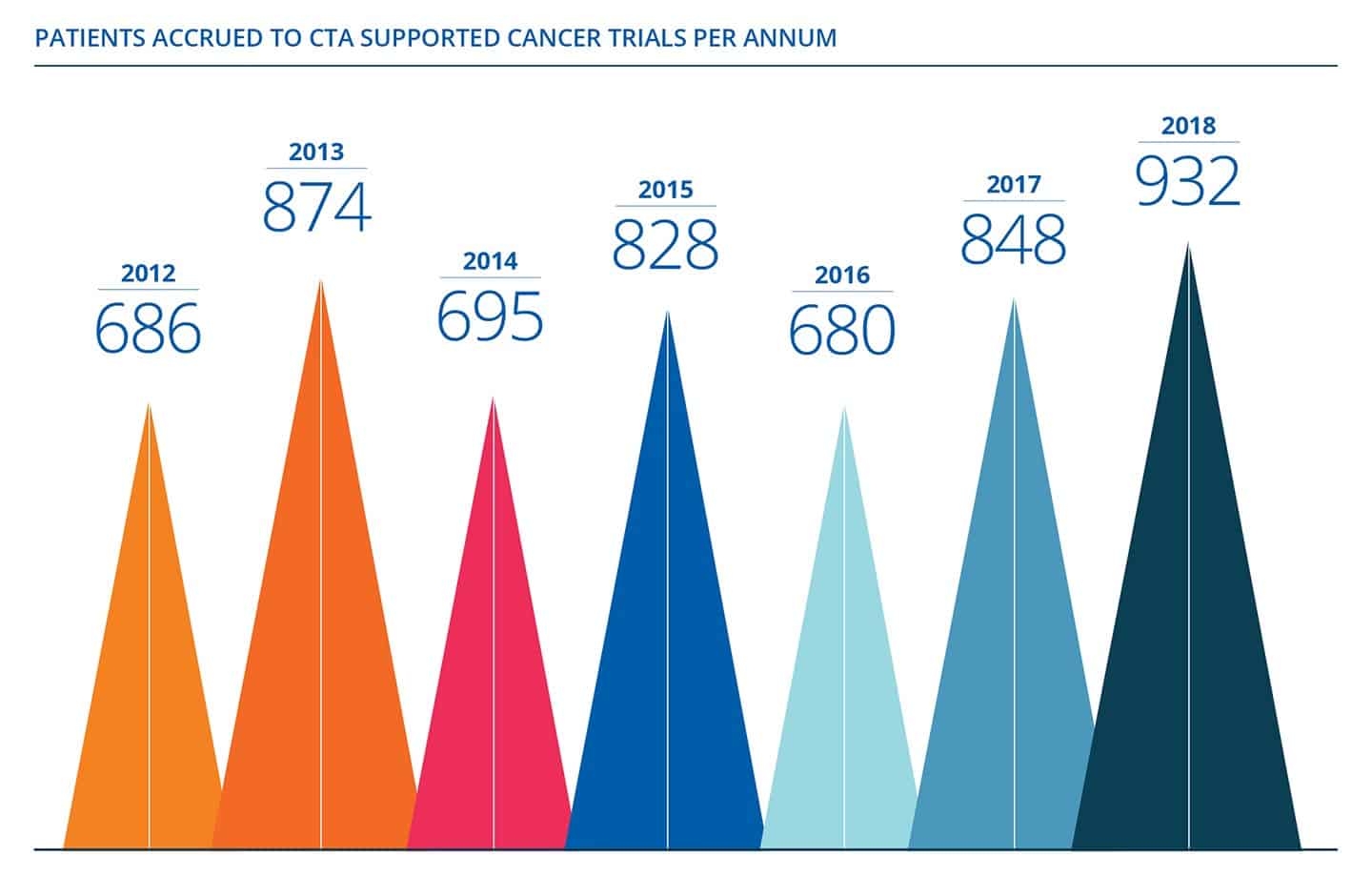
In a major milestone for Australian cancer patients, the not-for-profit, member-based organisation, Cancer Trials Australia (CTA), has recently marked the recruitment of the 10,000th oncology patient to clinical trials [...]
CTA Staff Photo Competition
Isolation is tough, especially when your workplace is a great place to be - the lack of incidental chatter and the enjoyment that comes from interacting with our workmates [...]
2019 Cancer Trials Australia Annual Report
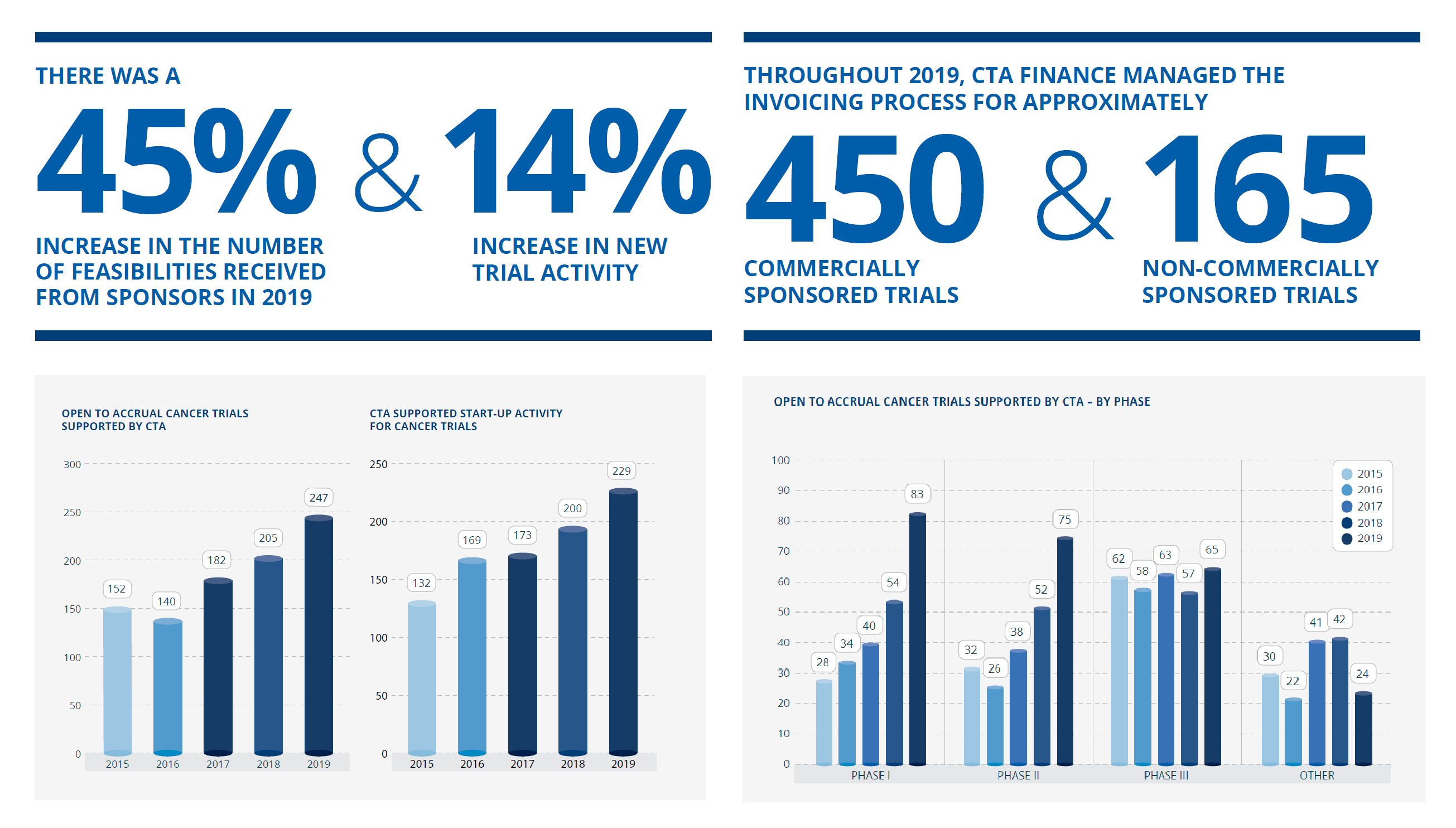
I am pleased to share with you the 2019 Cancer Trials Australia Annual Report. Download the 2019 Annual Report Here. The 2019 calendar year saw continued growth in both [...]
Pilot Phase 1 Oncology Clinical Trials Fellowship Program
Cancer Trials Australia (CTA) is pleased to announce that a Phase 1 Oncology Clinical Trials Fellowship Program will be piloted in Victoria throughout 2020 and 2021 in partnership with [...]
CTA CEO discusses key considerations for Chinese biotechs considering conducting clinical trials in Australia
Cancer Trials Australia’s CEO, Dr. Kurt Lackovic, was a panelist at the 12th annual ‘China Trials – Clinical Development Leaders’ summit’ in Shanghai this week. Other panelists included Jayden [...]
CTA announces partnership to drive Teletrials forward at Victorian study sites
Cancer Trials Australia (CTA) is pleased to announce a new project, to be delivered with the Victorian Comprehensive Cancer Centre (VCCC), providing Victorian sites with an exciting opportunity to [...]
Annual CTA Research Manager’s Day
Working closely with Marian Lieschke, Heike Raunow and Kylie Shackleton, CTA’s 2019 Research Manager’s Day was held on 17 October. 18 participants joined this highly productive forum from across [...]
CTA CEO presents at 3rd Annual Accelerating Clinical Trials in Asia conference
By 2021, the global market for clinical trials is anticipated to grow to $60Bn, a 75% increase over 2015. The largest contributor to this huge growth is the Asia-Pacific region.Cancer [...]
Hugely Successful 2019 CTA Team Development Day
CTA has experienced significant growth over recent years, which has accelerated throughout 2019. Dedicating time to team development activities not only helps to integrate new staff into the wider [...]
2018 Cancer Trials Australia Annual Report
I am pleased to share with you the 2018 Cancer Trials Australia Annual Report. Download the 2018 Annual Report Here. The 2018 calendar year saw growth in both CTA [...]
CTA and BeiGene profiled by Invest Victoria
Invest Victoria recently profiled the strength of interactions between Cancer Trials Australia and BeiGene, a rapidly growing Chinese biopharmaceutical company, with over 1,700 staff across four continents and more than [...]
CTA Collaborates With ShareRoot Ltd To Support Cancer Research
CTA has executed an MOU with ShareRoot Limited (ASX: SRO) to explore the application of ShareRoot’s MediaConsent platform as a tool to improve the dynamics of clinical trial recruitment, patient [...]
Coordinated Electronic Document Management for Victorian study sites
CTA announces partnership in delivering coordinated Electronic Document Management for Victorian study sites Cancer Trials Australia (CTA) is pleased to announce a new project funded by the Victorian Comprehensive [...]
CTA Expands Partnership with South West Healthcare
Due to an improved partnership between South West Healthcare (SWH) and CTA, cancer patients will have greater access to potentially life-saving therapies they may not have otherwise had access to. SWH medical oncologist Ian [...]
Calvary Central Districts Hospital joins CTA
Cancer Trials Australia is pleased to announce the addition of our 26th major healthcare provider member, Calvary Central Districts Hospital, located in South Australia. This partnership builds on Calvary's [...]
CTA to support VCCC Investigator Initiated Trial Program
Cancer Trials Australia (CTA) is pleased to announce execution of a Services Agreement with the VCCC to provide Site Management Services for the VCCC’s Investigator Initiated Trial Program. Professor [...]
2017 Cancer Trials Australia Annual Report
I am pleased to share the 2017 Cancer Trials Australia Annual Report. Download the 2017 Annual Report Here. 2017 saw expansion of CTA membership and significant growth in CTA services for [...]
CTA Celebrates 1,000 Commercially Sponsored Clinical Trials
Cancer Trials Australia celebrates their support of 1,000 commercially sponsored clinical trials and the recruitment of 8,000 patients, in conjunction with International Clinical Trials Day on May 21st, 2018 Since [...]