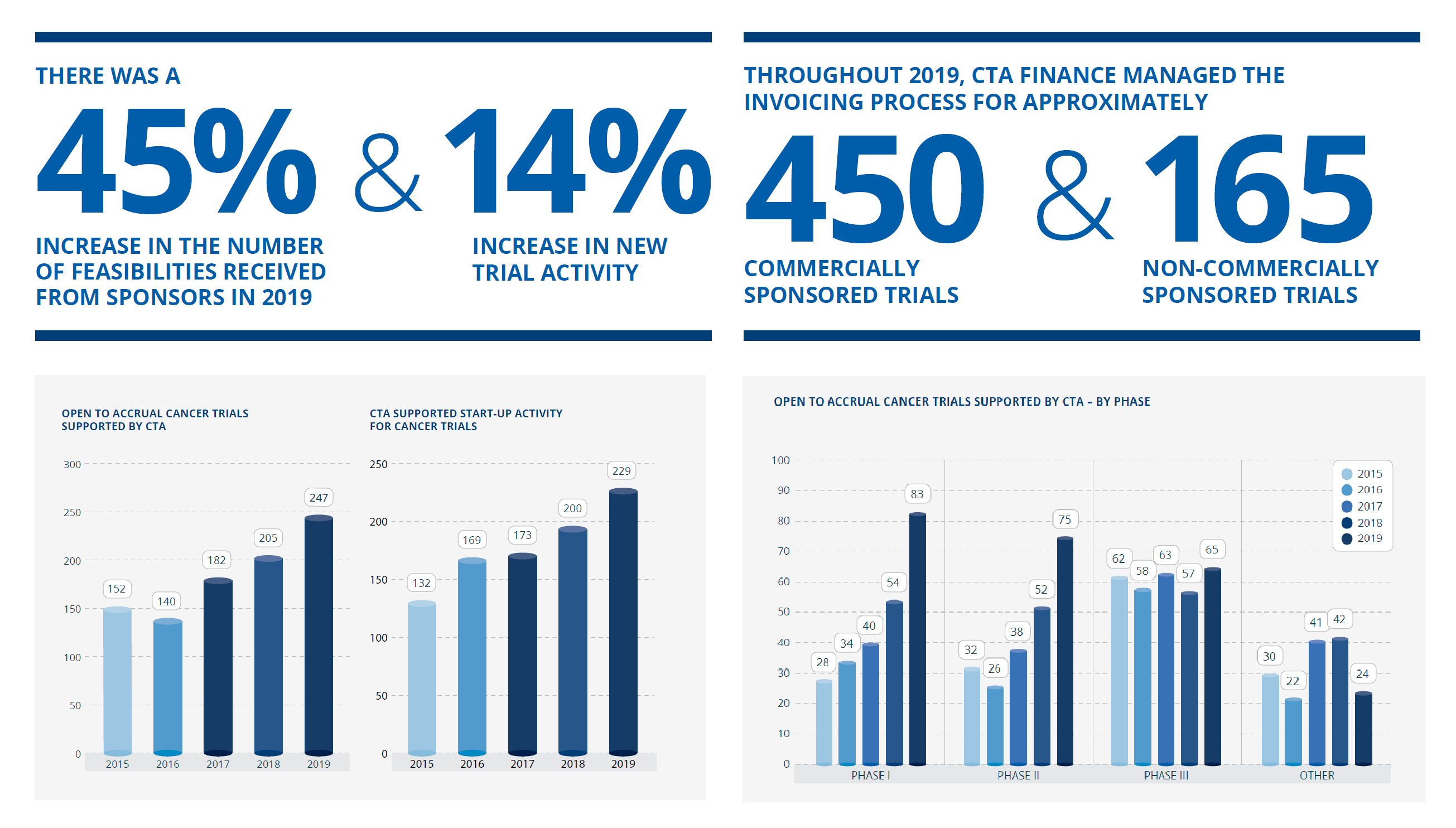
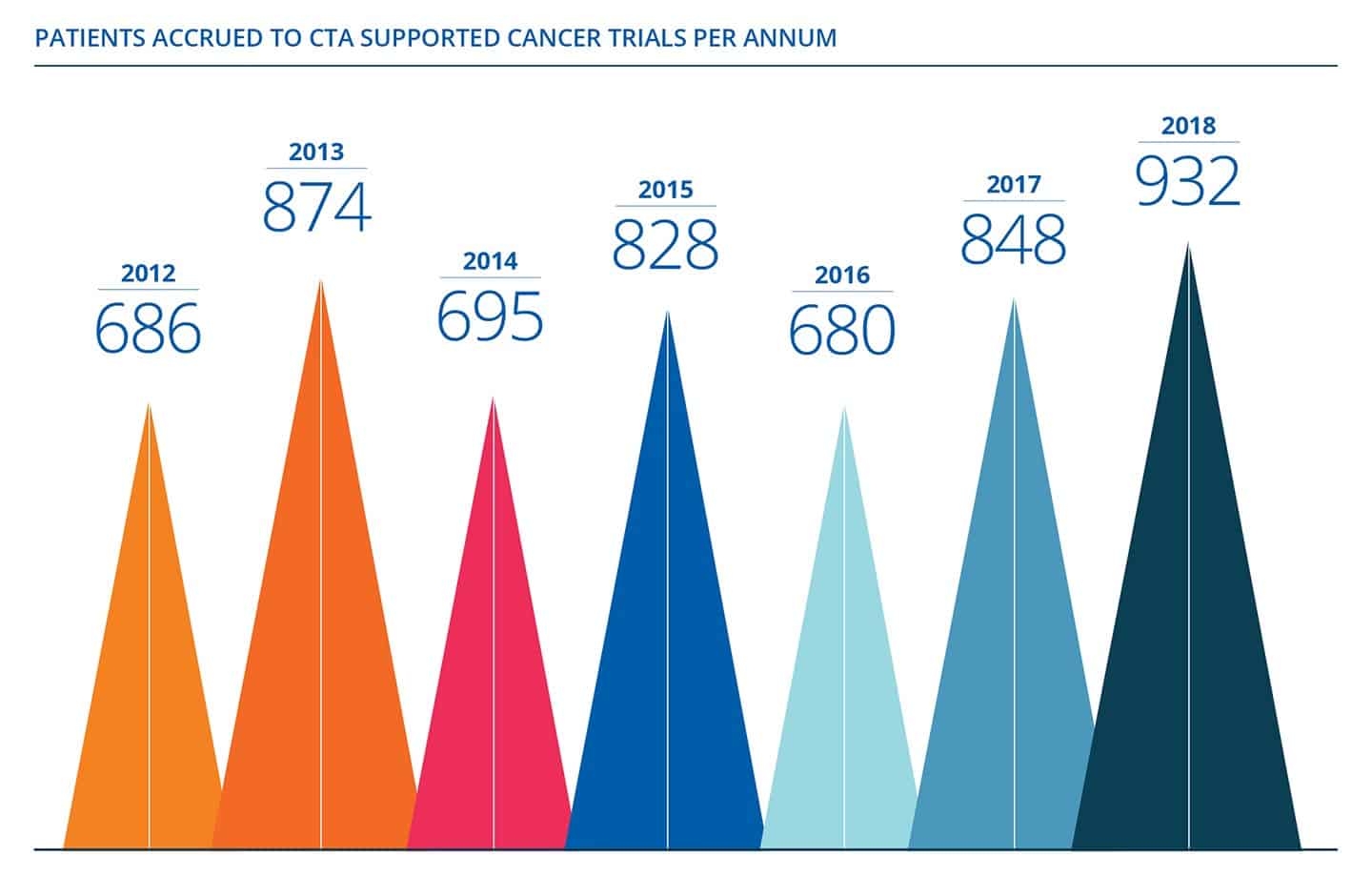
All Staff Stress and Resilience Workshop delivered by The Mind Room
In mid-November, CTA was pleased to offer a Stress and Resilience Workshop for all staff, delivered by Bailey Opie, a Performance Psychologist at The Mind Room. We were extremely [...]