I am pleased to share with you the 2019 Cancer Trials Australia Annual Report.
Download the 2019 Annual Report Here.
The 2019 calendar year saw continued growth in both CTA service provision and membership, welcoming Coffs Harbour Health Campus, and more recently, Auckland City Hospital and the Royal Children’s Hospital.
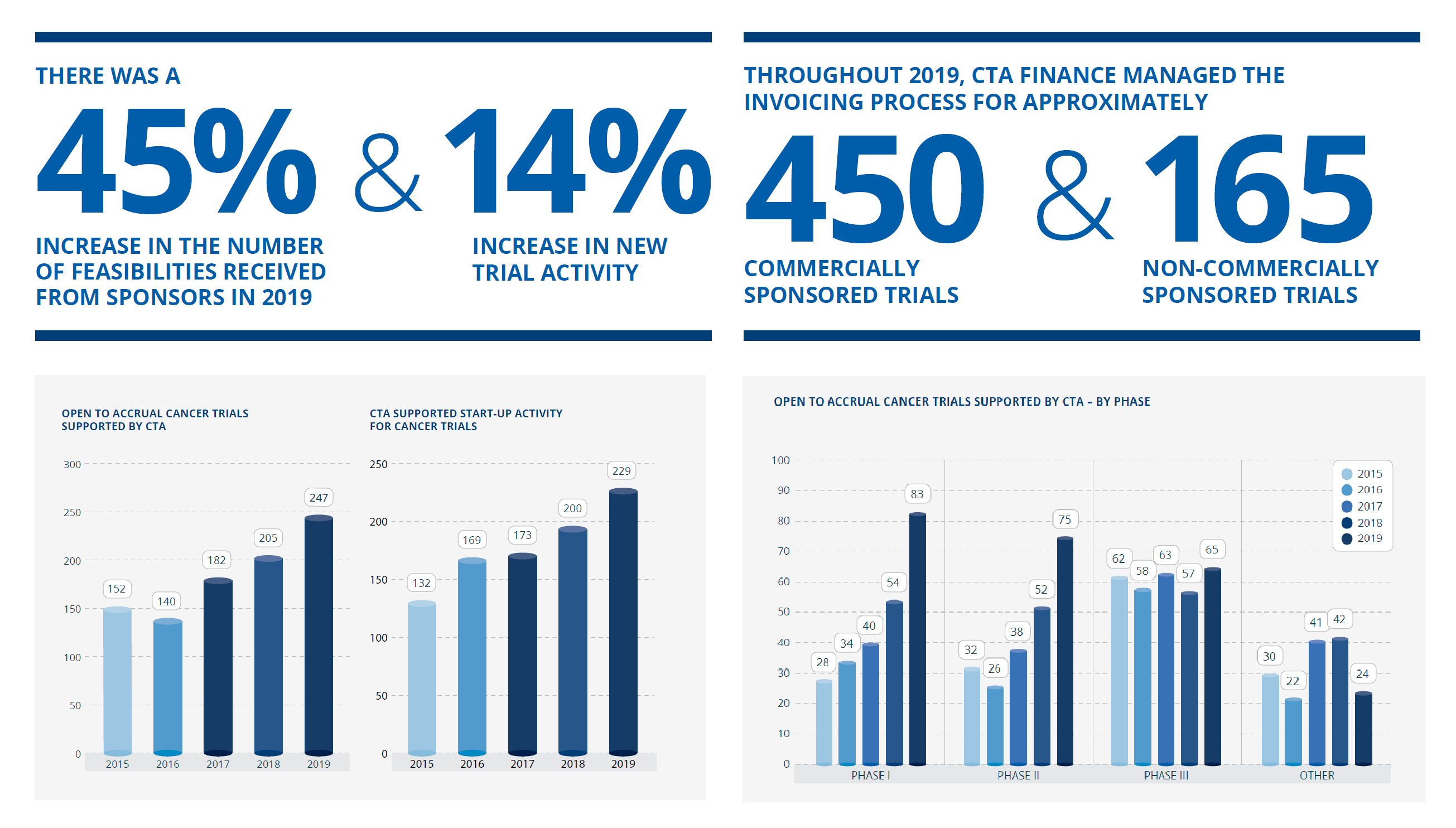
Building on the back of growth in previous years, almost all CTA service metrics improved further in 2019; feasibilities grew 45% and new trial activity increased 14%. Significant expansion continued in non-oncology submissions. The CTA Finance team issued in excess of 5,000 invoices on behalf of our Members, and almost $27M was distributed to CTA Members, up 15% on the previous year.
Our key programs in 2019 included (i) announcing a Phase 1 Oncology Clinical Trials Fellowship Program to be piloted in Victoria, in partnership with the Victorian Government through the Victorian Cancer Agency (VCA), (ii) on-going strategic investment in our information management systems, (ii) leading the implementation of an electronic document management system (SiteDocs Portal) across 17 Victorian hospitals, (iii) ensuring a strong sector voice in national and international forums, and (iv) continuing to enhance linkages across metro and regional hospitals.
Cancer Trials Australia remains well positioned to continue to support clinical trial activity across our growing membership, helping to ensure Australia remains a destination of choice for clinical trials and securing earlier access to novel therapies for Australian patients.
I would like to take the opportunity to thank all CTA staff, as well as our network Member personnel, for all the hard work they undertake that is essential to enable the CTA network to flourish.
For a hard copy of the report please contact [email protected]
Kurt Lackovic
Chief Executive Officer